Embracing the Future: FDA’s New Draft Guidelines and the Role of Digital Platforms in Prescription Drug Use
A pivotal moment in the convergence of healthcare and technology
In the ever-evolving landscape of healthcare technology, the FDA’s recent guidelines on prescription drug use related software mark a significant step towards harnessing digital solutions to enhance patient outcomes and safety. These guidelines, aimed at promoting innovation while ensuring regulatory clarity, have opened doors for digital platforms like Medisafe to emerge as pivotal partners for pharmaceutical companies striving to adhere to these standards at scale.
Understanding the FDA’s Guidelines
The FDA’s guidelines reflect a proactive approach to regulating software that supports the use of prescription drugs. They distinguish between software that may impact clinical decision-making and software that provides general health-related information. This distinction helps streamline the regulatory process, ensuring that tools critical to patient care meet rigorous safety and effectiveness standards, while allowing for flexibility in non-clinical software that supports medication adherence and management.
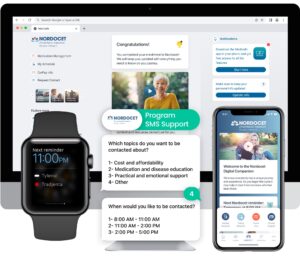 The Role of Digital Platforms
The Role of Digital Platforms
Digital health platforms, such as Medisafe, play a crucial role in the pharmaceutical ecosystem by leveraging technology to enhance patient engagement and medication adherence. These platforms integrate seamlessly into patients’ daily lives, offering personalized medication reminders, educational content, and progress tracking. By empowering patients with information and support, digital platforms contribute significantly to improved treatment adherence and health outcomes.
Meeting Regulatory Standards at Scale
For pharmaceutical companies navigating the complexities of compliance with the FDA’s guidelines, partnering with a proven digital platform like Medisafe offers distinct advantages. These platforms are designed to meet stringent regulatory requirements, ensuring data security, privacy, and adherence to FDA guidelines throughout their operations. Medisafe, for instance, implements robust measures to safeguard patient information and adheres to industry best practices in software development and data management.
Enhancing Patient Outcomes
The primary goal of integrating digital platforms into the healthcare ecosystem is to improve patient outcomes. By providing real-time support and feedback, Medisafe helps patients stay on track with their prescribed therapies, thereby reducing the risks associated with non-adherence. Through personalized notifications and educational content, patients are empowered to make informed decisions about their health, fostering a collaborative approach between healthcare providers, patients, and pharmaceutical companies.
Scalability and Impact
One of the key advantages of digital platforms is their scalability. Medisafe, for example, has a global reach, making it an ideal partner for pharmaceutical companies looking to deploy solutions across diverse patient populations. Whether managing chronic conditions or facilitating clinical trials, these platforms can adapt to meet the unique needs of different therapeutic areas and patient demographics, all while maintaining adherence to regulatory standards.
Future Outlook
As the healthcare industry continues to embrace digital transformation, the role of digital platforms in medication management and adherence will only grow more critical. The FDA’s guidelines pave the way for innovation in software solutions that support safe and effective use of prescription drugs, offering opportunities for collaboration between pharmaceutical companies and technology providers like Medisafe.
The FDA’s new guidelines on prescription drug use related software mark a pivotal moment in the convergence of healthcare and technology. Digital platforms such as Medisafe not only facilitate adherence to these guidelines but also enhance patient engagement and outcomes through personalized support and data-driven insights. By embracing these innovations, pharmaceutical companies can not only meet regulatory standards but also deliver superior patient care on a global scale.
As we move forward, the synergy between regulatory frameworks and digital health solutions promises a future where patient-centric care and technological innovation intersect seamlessly, shaping a healthier tomorrow for all.
Related: 6 Ways Patients will Benefit from the FDA’s PDURS Guidelines
Let’s Talk About Medisafe and PDURS:
